Background:
Primary central nervous system lymphoma (PCNSL) is a rare, aggressive B-cell malignancy. Despite the possibility of a cure, the prognosis remains poor. The standard treatment includes high-dose methotrexate and rituximab, followed by consolidation with either whole-brain radiation therapy or autologous stem cell transplantation (ASCT). However, there is still no clear consensus on the best treatment approach. To improve outcomes, we incorporated thiotepa chemotherapy, which has shown promising activity in inhibiting tumor growth by disrupting DNA bonds, into the regimen. Additionally, we introduced orelabrutinib, a novel and potent BTK inhibitor with high cerebrospinal fluid concentrations, which has demonstrated efficacy in treating PCNSL. In light of these findings, we conducted a clinical study to evaluate the efficacy and safety of a combination regimen of thiotepa, orelabrutinib, and methotrexate with rituximab (R-MTO) followed by autologous hematopoietic stem cell transplantation (HSCT) in patients with PCNSL.
Methods:
This is an ongoing, single-center, single-arm, open-label study conducted between April 2022 and July 2023. The study enrolled patients aged 18-80 years diagnosed with PCNSL. The patients received 5-8 cycles of R-MTO regimen as induction therapy, consisting of rituximab 375 mg/m 2 iv on day 0, MTX 3.5 g/m 2 iv on day1, thiotepa, 30-40 mg/m 2 on day 4 and orelabrutinib 150 mg qd po for 3 weeks per cycle). After completing the R-MTO therapy, patients were proposed to receive orelabrutinib monotherapy as maintenance treatment for up to 2 years. The efficacy was assessed by MRI/PET per the International PCNSL Collaborative Group (IPCG) criteria.
Patients who did not experience disease progression subsequently underwent consolidation treatment with high-dose chemotherapy (HCT) plus ASCT. The HCT regimen involved busulfan 3.2 mg/kg on days -5, -4, thiotepa 250 mg/m 2 on days -7 and -6, cytarabine 2g/m 2 q12h on days -3 and -2, followed by reinfusion of stem cells on day 0. The primary endpoint of the study was progression-free survival (PFS) at 2 years, evaluated by intention-to-treat (ITT). Secondary endpoints included complete response (CR) rate, overall response rate (ORR), overall survival (OS) and toxicities.
Results:
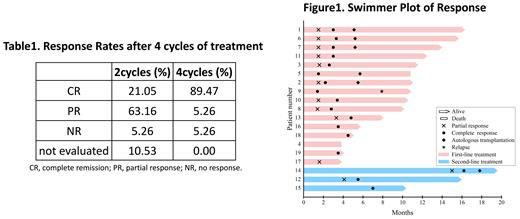
Between April 2022 and July 2023, a total of 19 patients with PCNSL were enrolled in our study, including 16 who were newly diagnosed and had not received any prior treatment. The median age of the patients was 62 years, ranging from 31 to 72, with 9 out of 19 (47.37%) being male. By the data cutoff, all the patients had completed 4 cycles of treatment. Additionally, 8 out of 19 (42.11%) patients had completed six cycles of treatment, and 2 out of 19 (10.52%) patients had completed 7 to 8 cycles of treatment. The ORR was 94.73% (18/19), and the CR rate was 89.47% (17/19) after 4 cycles of treatment. However, one patient with a P53 mutation progressed after 2 cycles of treatment and unfortunately died 3.8 months after diagnosis. Among the six patients who underwent autologous HSCT, all of them (100%) remained in CR. One patient died due to a COVID-19 infection after 6 cycles of R-MTO and ASCT. The most common hematological adverse events associated with the R-MTO regimen were mild to moderate (grade 1-2) decreases in neutrophil count, which typically resolved within a few days.
Conclusions:
The R-MTO induction treatment has demonstrated notable efficacy in achieving higher response rates among patients with PCNSL, and the associated adverse events are manageable.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal